How Many Atoms Of Potassium Makeup 2 Mole
Chapter 3. Composition of Substances and Solutions
iii.i Formula Mass and the Mole Concept
Learning Objectives
By the terminate of this section, yous will be able to:
- Calculate formula masses for covalent and ionic compounds
- Define the amount unit mole and the related quantity Avogadro's number
Explain the relation betwixt mass, moles, and numbers of atoms or molecules, and perform calculations deriving these quantities from one another
We can argue that modern chemical science began when scientists started exploring the quantitative as well as the qualitative aspects of chemistry. For case, Dalton'due south atomic theory was an try to explain the results of measurements that immune him to calculate the relative masses of elements combined in diverse compounds. Understanding the human relationship between the masses of atoms and the chemical formulas of compounds allows us to quantitatively describe the limerick of substances.
Formula Mass
In an before affiliate, we described the development of the atomic mass unit of measurement, the concept of average diminutive masses, and the use of chemical formulas to represent the elemental makeup of substances. These ideas tin exist extended to calculate the formula mass of a substance by summing the average atomic masses of all the atoms represented in the substance's formula.
Formula Mass for Covalent Substances
For covalent substances, the formula represents the numbers and types of atoms composing a single molecule of the substance; therefore, the formula mass may be correctly referred to as a molecular mass. Consider chloroform (CHCl3), a covalent compound in one case used as a surgical coldhearted and now primarily used in the production of the "anti-stick" polymer, Teflon. The molecular formula of chloroform indicates that a single molecule contains one carbon cantlet, one hydrogen atom, and iii chlorine atoms. The average molecular mass of a chloroform molecule is therefore equal to the sum of the average atomic masses of these atoms. Effigy 1 outlines the calculations used to derive the molecular mass of chloroform, which is 119.37 amu.
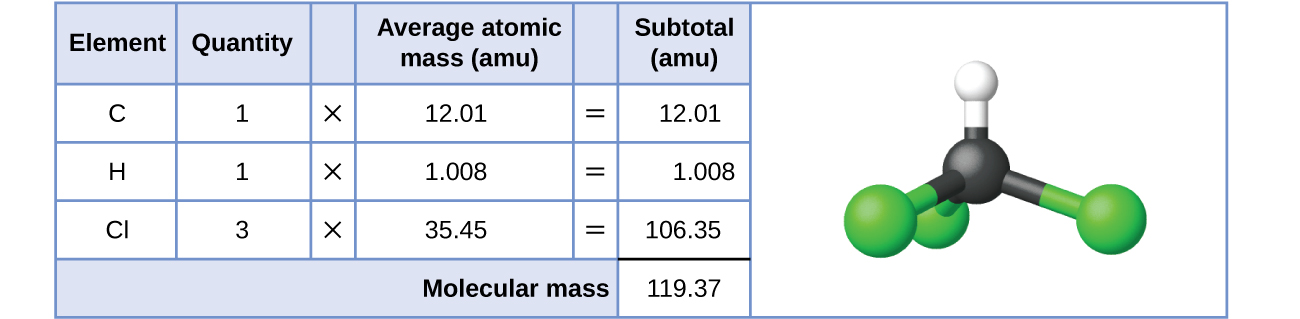
Besides, the molecular mass of an aspirin molecule, CnineH8Oiv, is the sum of the diminutive masses of nine carbon atoms, eight hydrogen atoms, and iv oxygen atoms, which amounts to 180.15 amu (Figure ii).
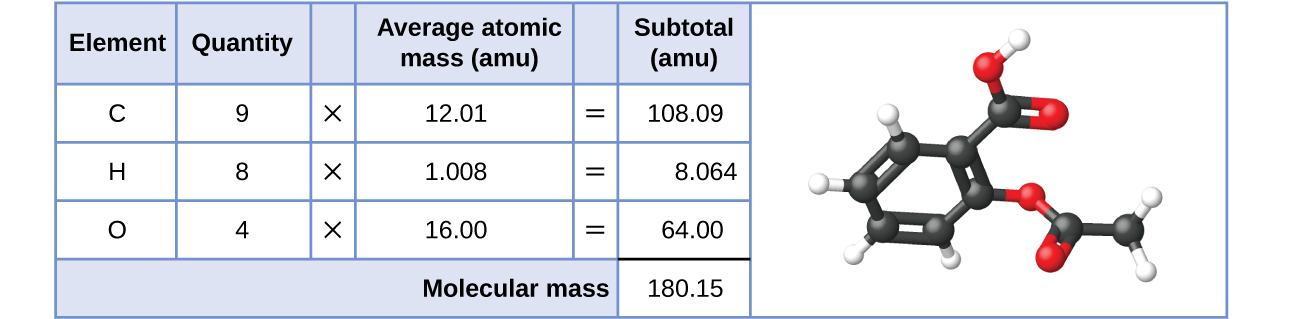
Case 1
Computing Molecular Mass for a Covalent Compound
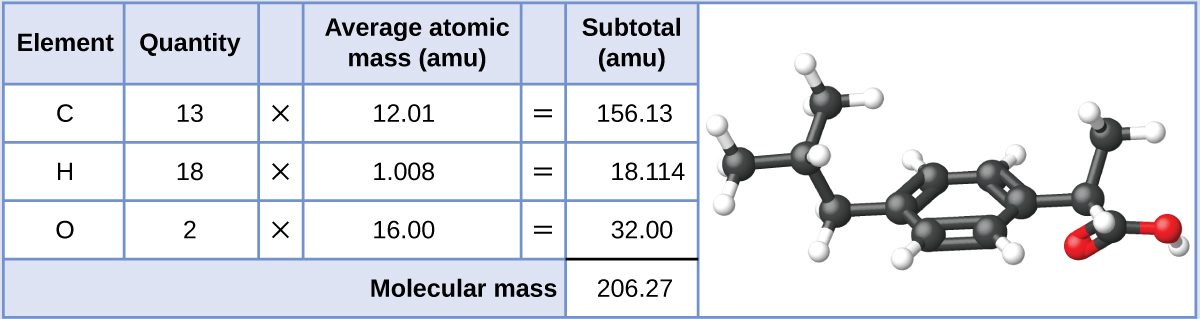
Ibuprofen, CxiiiHxviiiO2, is a covalent chemical compound and the agile ingredient in several pop nonprescription pain medications, such as Advil and Motrin. What is the molecular mass (amu) for this compound?
Solution
Molecules of this compound are comprised of thirteen carbon atoms, xviii hydrogen atoms, and 2 oxygen atoms. Following the approach described in a higher place, the boilerplate molecular mass for this compound is therefore:
Check Your Learning
Acetaminophen, CeightHnineNO2, is a covalent compound and the agile ingredient in several popular nonprescription pain medications, such as Tylenol. What is the molecular mass (amu) for this compound?
Formula Mass for Ionic Compounds
Ionic compounds are equanimous of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic chemical compound is calculated in the same mode as the formula mass for covalent compounds: past summing the average atomic masses of all the atoms in the compound's formula. Keep in listen, all the same, that the formula for an ionic chemical compound does not represent the composition of a discrete molecule, then it may not correctly be referred to as the "molecular mass."
As an example, consider sodium chloride, NaCl, the chemic name for common salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl−, combined in a one:ane ratio. The formula mass for this chemical compound is computed as 58.44 amu (see Effigy 3).
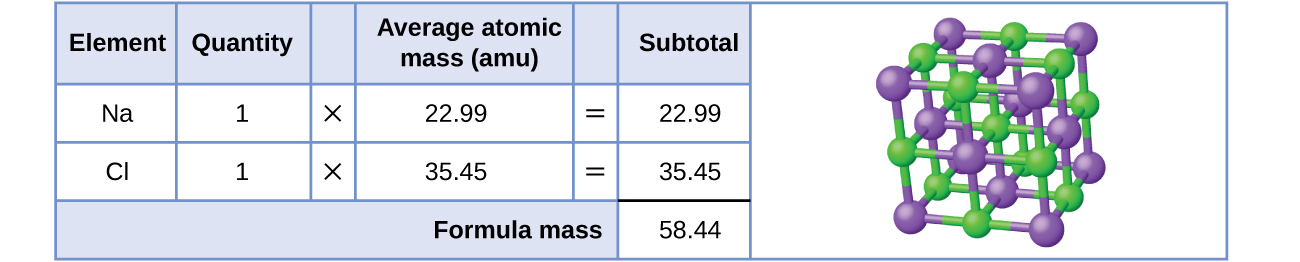
Note that the boilerplate masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. This approach is perfectly acceptable when computing the formula mass of an ionic compound. Fifty-fifty though a sodium cation has a slightly smaller mass than a sodium atom (since it is missing an electron), this difference will be offset by the fact that a chloride anion is slightly more massive than a chloride atom (due to the actress electron). Moreover, the mass of an electron is negligibly small-scale with respect to the mass of a typical cantlet. Fifty-fifty when computing the mass of an isolated ion, the missing or additional electrons can more often than not exist ignored, since their contribution to the overall mass is negligible, reflected only in the nonsignificant digits that volition be lost when the computed mass is properly rounded. The few exceptions to this guideline are very lite ions derived from elements with precisely known atomic masses.
Example 2
Computing Formula Mass for an Ionic Compound
Aluminum sulfate, Al2(SO4)iii, is an ionic compound that is used in the manufacture of paper and in various water purification processes. What is the formula mass (amu) of this compound?
Solution
The formula for this compound indicates information technology contains Aliii+ and SOiv 2− ions combined in a two:three ratio. For purposes of calculating a formula mass, it is helpful to rewrite the formula in the simpler format, AliiSiiiO12. Following the arroyo outlined to a higher place, the formula mass for this compound is calculated as follows:
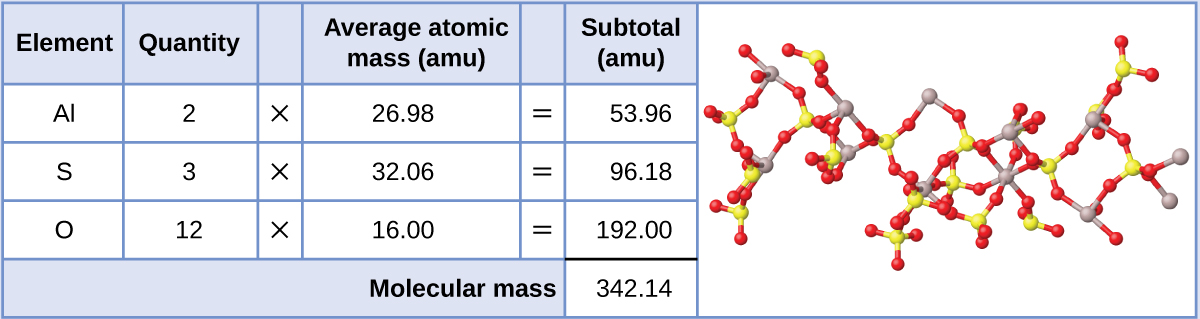
Check Your Learning
Calcium phosphate, Ca3(POiv)two, is an ionic chemical compound and a common anti-caking agent added to food products. What is the formula mass (amu) of calcium phosphate?
The Mole
The identity of a substance is defined not only by the types of atoms or ions it contains, but by the quantity of each type of atom or ion. For example, water, H2O, and hydrogen peroxide, H2O2, are alike in that their corresponding molecules are composed of hydrogen and oxygen atoms. Even so, considering a hydrogen peroxide molecule contains two oxygen atoms, every bit opposed to the water molecule, which has only one, the 2 substances exhibit very dissimilar backdrop. Today, we possess sophisticated instruments that allow the direct measurement of these defining microscopic traits; however, the same traits were originally derived from the measurement of macroscopic properties (the masses and volumes of bulk quantities of matter) using relatively simple tools (balances and volumetric glassware). This experimental arroyo required the introduction of a new unit for amount of substances, the mole, which remains indispensable in modern chemical science.
The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter. A mole is defined as the amount of substance containing the same number of discrete entities (such every bit atoms, molecules, and ions) equally the number of atoms in a sample of pure 12C weighing exactly 12 chiliad. One Latin connotation for the word "mole" is "large mass" or "bulk," which is consequent with its employ every bit the name for this unit. The mole provides a link between an easily measured macroscopic property, bulk mass, and an extremely of import fundamental property, number of atoms, molecules, and then forth.
The number of entities composing a mole has been experimentally determined to be half-dozen.02214179 × 1023, a fundamental constant named Avogadro's number (NorthwardA ) or the Avogadro constant in honour of Italian scientist Amedeo Avogadro. This constant is properly reported with an explicit unit of measurement of "per mole," a conveniently rounded version being six.022 × 1023/mol.
Consistent with its definition as an amount unit, i mole of whatsoever element contains the same number of atoms every bit 1 mole of any other element. The masses of 1 mole of unlike elements, however, are different, since the masses of the individual atoms are drastically different. The tooth mass of an element (or compound) is the mass in grams of 1 mole of that substance, a holding expressed in units of grams per mole (g/mol) (see Effigy 4).

Considering the definitions of both the mole and the diminutive mass unit of measurement are based on the aforementioned reference substance, 12C, the tooth mass of whatever substance is numerically equivalent to its diminutive or formula weight in amu. Per the amu definition, a unmarried 12C cantlet weighs 12 amu (its diminutive mass is 12 amu). According to the definition of the mole, 12 one thousand of 12C contains i mole of 12C atoms (its molar mass is 12 thousand/mol). This human relationship holds for all elements, since their diminutive masses are measured relative to that of the amu-reference substance, 12C. Extending this principle, the tooth mass of a compound in grams is also numerically equivalent to its formula mass in amu (Effigy 5).

| Element | Average Diminutive Mass (amu) | Molar Mass (g/mol) | Atoms/Mole |
|---|---|---|---|
| C | 12.01 | 12.01 | 6.022 × x23 |
| H | 1.008 | 1.008 | vi.022 × x23 |
| O | 16.00 | 16.00 | 6.022 × 1023 |
| Na | 22.99 | 22.99 | 6.022 × 1023 |
| Cl | 35.45 | 33.45 | 6.022 × 1023 |
| Table i. | |||
While atomic mass and molar mass are numerically equivalent, proceed in mind that they are vastly dissimilar in terms of calibration, every bit represented by the vast difference in the magnitudes of their corresponding units (amu versus g). To capeesh the enormity of the mole, consider a pocket-sized drib of water weighing about 0.03 g (see Figure 6). Although this represents only a tiny fraction of i mole of h2o (~xviii g), it contains more than h2o molecules than can be clearly imagined. If the molecules were distributed every bit amid the roughly seven billion people on earth, each person would receive more than than 100 billion molecules.


The mole is used in chemistry to represent 6.022 × 1023 of something, only it can be difficult to conceptualize such a large number. Sentry this video and then complete the "Think" questions that follow. Explore more near the mole by reviewing the data under "Dig Deeper."
The relationships between formula mass, the mole, and Avogadro's number can be applied to compute various quantities that describe the limerick of substances and compounds. For example, if we know the mass and chemical limerick of a substance, we can decide the number of moles and calculate number of atoms or molecules in the sample. As well, if we know the number of moles of a substance, we can derive the number of atoms or molecules and calculate the substance's mass.
Example 3
Deriving Moles from Grams for an Element
According to nutritional guidelines from the U.s.a. Department of Agronomics, the estimated average requirement for dietary potassium is 4.vii yard. What is the estimated average requirement of potassium in moles?
Solution
The mass of One thousand is provided, and the corresponding corporeality of Yard in moles is requested. Referring to the periodic tabular array, the atomic mass of K is 39.10 amu, and so its molar mass is 39.x g/mol. The given mass of K (four.7 g) is a flake more one-tenth the molar mass (39.ten g), so a reasonable "ballpark" estimate of the number of moles would be slightly greater than 0.1 mol.
The molar amount of a substance may be calculated by dividing its mass (chiliad) past its molar mass (grand/mol):

The gene-label method supports this mathematical approach since the unit "g" cancels and the reply has units of "mol:"
[latex]iv.7 \dominion[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{G} (\frac{\text{mol One thousand}}{39.x \rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{1000}}) = 0.12 \;\text{mol Thou}[/latex]
The calculated magnitude (0.12 mol Thou) is consistent with our ballpark expectation, since it is a bit greater than 0.1 mol.
Bank check Your Learning
Beryllium is a light metal used to fabricate transparent X-ray windows for medical imaging instruments. How many moles of Be are in a thin-foil window weighing three.24 k?
Example iv
Deriving Grams from Moles for an Chemical element
A liter of air contains 9.two × x−4 mol argon. What is the mass of Ar in a liter of air?
Solution
The molar amount of Ar is provided and must be used to derive the corresponding mass in grams. Since the amount of Ar is less than 1 mole, the mass will be less than the mass of 1 mole of Ar, approximately 40 g. The molar amount in question is approximately 1-one thousandth (~10−3) of a mole, and then the corresponding mass should be roughly 1-1 thousandth of the molar mass (~0.04 1000):

In this example, logic dictates (and the cistron-label method supports) multiplying the provided corporeality (mol) by the molar mass (g/mol):
[latex]9.2 \times ten^{-iv} \;\rule[0.5ex]{1.75em}{0.1ex}\hspace{-1.75em}\text{mol} \;\text{Ar} (\frac{39.95 \;\text{g}}{\rule[0.25ex]{one.25em}{0.1ex}\hspace{-ane.25em}\text{mol} \;\text{Ar}}) = 0.037 \;\text{yard Ar}[/latex]
The effect is in understanding with our expectations, around 0.04 g Ar.
Check Your Learning
What is the mass of two.561 mol of gold?
Example v
Deriving Number of Atoms from Mass for an Element
Copper is commonly used to fabricate electrical wire (Effigy 7). How many copper atoms are in v.00 k of copper wire?

Solution
The number of Cu atoms in the wire may be conveniently derived from its mass by a two-footstep computation: first calculating the molar amount of Cu, and and then using Avogadro's number (NA ) to convert this tooth amount to number of Cu atoms:

Considering that the provided sample mass (five.00 one thousand) is a petty less than 1-tenth the mass of ane mole of Cu (~64 g), a reasonable estimate for the number of atoms in the sample would exist on the order of one-10th NA , or approximately 1022 Cu atoms. Carrying out the two-step computation yields:
[latex]five.00 \;\dominion[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{Cu} (\frac{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-ane.25em}\text{mol} \;\text{Cu}}{63.55 \dominion[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}})(\frac{half dozen.022 \times 10^{23} \;\text{atoms}}{\dominion[0.25ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{mol}}) = iv.74 \times 10^{22} \;\text{atoms of copper}[/latex]
The factor-label method yields the desired cancellation of units, and the computed result is on the order of 1022 as expected.
Check Your Learning
A prospector panning for golden in a river collects xv.00 g of pure gold. How many Au atoms are in this quantity of gold?
Respond:
4.586 × 1022 Au atoms
Example 6
Deriving Moles from Grams for a Chemical compound
Our bodies synthesize poly peptide from amino acids. One of these amino acids is glycine, which has the molecular formula C2H5O2N. How many moles of glycine molecules are contained in 28.35 1000 of glycine?
Solution
We can derive the number of moles of a compound from its mass following the same process we used for an chemical element in Example iii:
The molar mass of glycine is required for this adding, and it is computed in the same fashion as its molecular mass. I mole of glycine, C2H5O2N, contains two moles of carbon, five moles of hydrogen, 2 moles of oxygen, and ane mole of nitrogen:
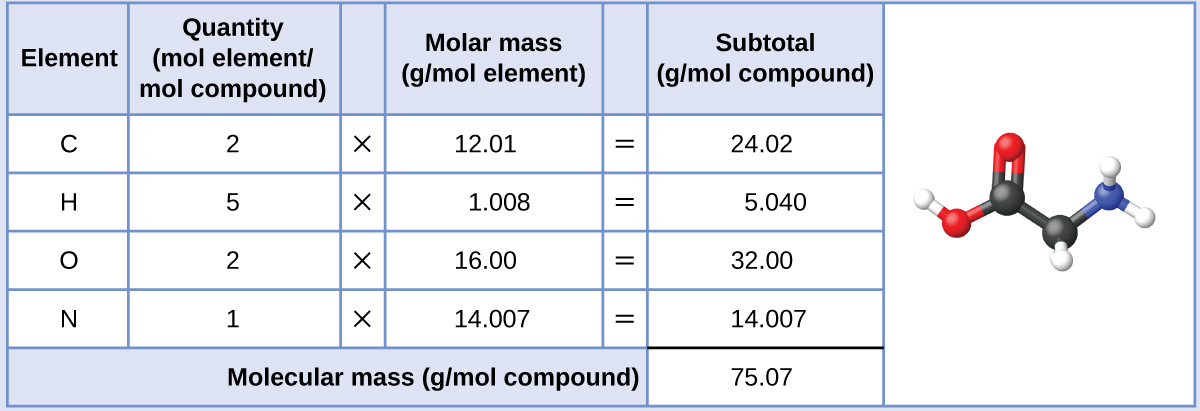
The provided mass of glycine (~28 yard) is a bit more than one-third the tooth mass (~75 chiliad/mol), so we would look the computed outcome to be a bit greater than one-third of a mole (~0.33 mol). Dividing the chemical compound'due south mass by its molar mass yields:
[latex]28.35 \;\rule[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;\text{glycine} \;(\frac{\text{mol glycine}}{75.07 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}}) = 0.378 \;\text{mol glycine}[/latex]
This consequence is consequent with our crude estimate.
Bank check Your Learning
How many moles of sucrose, C12H22O11, are in a 25-g sample of sucrose?
Instance vii
Deriving Grams from Moles for a Chemical compound
Vitamin C is a covalent compound with the molecular formula CsixHeightOhalf-dozen. The recommended daily dietary allowance of vitamin C for children aged 4–8 years is 1.42 × 10−4 mol. What is the mass of this assart in grams?
Solution
As for elements, the mass of a chemical compound can be derived from its molar amount as shown:

The molar mass for this compound is computed to be 176.124 one thousand/mol. The given number of moles is a very small-scale fraction of a mole (~10−4 or one-ten thousandth); therefore, we would expect the corresponding mass to exist about one-ten thousandth of the molar mass (~0.02 k). Performing the calculation, we go:
[latex]1.42 \times 10^{-4} \;\rule[0.5ex]{1.75em}{0.1ex}\hspace{-ane.75em}\text{mol} \;\text{vitamin C} (\frac{176.124 \text{k}}{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-1.25em}\text{mol} \;\text{vitamin C}}) = 0.0250 \;\text{g vitamin C}[/latex]
This is consistent with the predictable result.
Check Your Learning
What is the mass of 0.443 mol of hydrazine, North2H4?
Example 8
Deriving the Number of Atoms and Molecules from the Mass of a Compound
A packet of an artificial sweetener contains 40.0 mg of saccharin (C7H5NO3Southward), which has the structural formula:
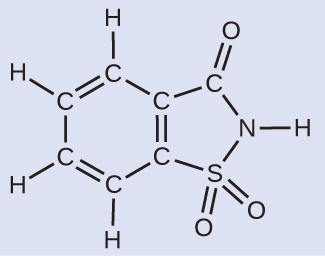
Given that saccharin has a molar mass of 183.18 chiliad/mol, how many saccharin molecules are in a 40.0-mg (0.0400-g) sample of saccharin? How many carbon atoms are in the same sample?
Solution
The number of molecules in a given mass of chemical compound is computed by first deriving the number of moles, as demonstrated in Example 6, then multiplying by Avogadro's number:
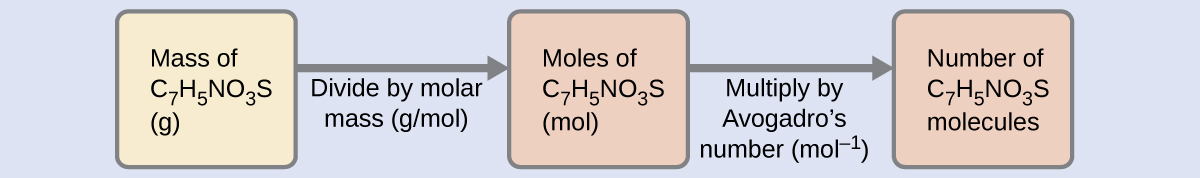
Using the provided mass and molar mass for saccharin yields:
[latex]0.0400 \;\dominion[0.5ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{thousand} \;\text{C}_7\text{H}_5\text{NO}_3\text{S} (\frac{\rule[0.25ex]{1.25em}{0.1ex}\hspace{-ane.25em}\text{mol} \;\text{C}_7\text{H}_5\text{NO}_3\text{S}}{183.18 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{thou} \;\text{C}_7\text{H}_5\text{NO}_3\text{South}}) (\frac{six.022 \times x^{23} \;\text{C}_7\text{H}_5\text{NO}_3\text{S} \;\text{molecules}}{ane\;\dominion[0.25ex]{one.25em}{0.1ex}\hspace{-1.25em}\text{mol} \;\text{C}_7\text{H}_5\text{NO}_3\text{South}})[/latex] [latex]= 1.31 \times 10^{20} \;\text{C}_7\text{H}_5\text{NO}_3\text{S} \;\text{molecules}[/latex]
The compound's formula shows that each molecule contains seven carbon atoms, and so the number of C atoms in the provided sample is:
[latex]1.31 \times x^{twenty} \;\text{C}_7\text{H}_5\text{NO}_3\text{S molecules} \;(\frac{seven \;\text{C atoms}}{one \;\text{C}_7\text{H}_5\text{NO}_3\text{Due south molecule}}) = 9.20 \times 10^{21} \;\text{C atoms}[/latex]
Check Your Learning
How many C4H10 molecules are contained in nine.213 chiliad of this chemical compound? How many hydrogen atoms?
Respond:
9.545 × 1022 molecules C4 Hx; ix.545 × 1023 atoms H
Counting Neurotransmitter Molecules in the Brain
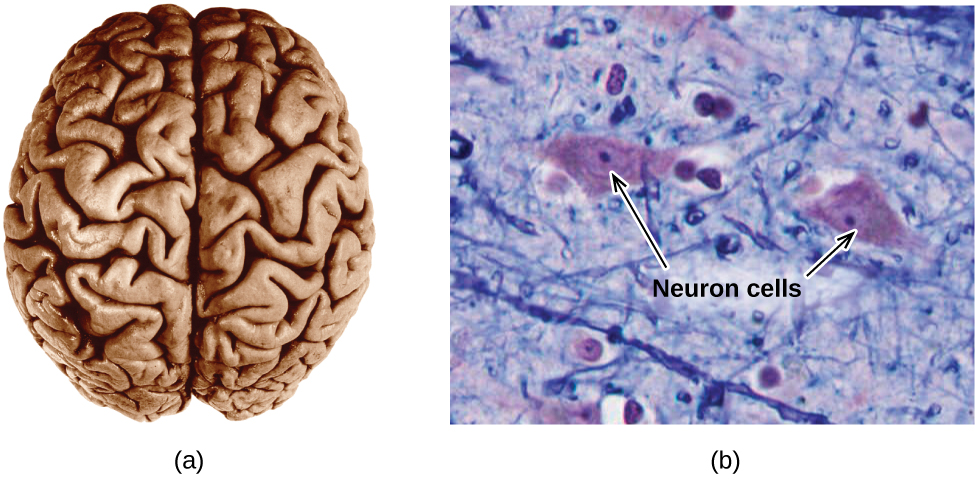
The brain is the control center of the central nervous organization (Effigy eight). Information technology sends and receives signals to and from muscles and other internal organs to monitor and control their functions; information technology processes stimuli detected by sensory organs to guide interactions with the external world; and it houses the complex physiological processes that give rise to our intellect and emotions. The broad field of neuroscience spans all aspects of the structure and role of the central nervous arrangement, including research on the beefcake and physiology of the encephalon. Great progress has been fabricated in brain research over the past few decades, and the BRAIN Initiative, a federal initiative announced in 2013, aims to accelerate and capitalize on these advances through the concerted efforts of diverse industrial, academic, and government agencies (more than details available at www.whitehouse.gov/share/brain-initiative).
Specialized cells called neurons transmit information between different parts of the central nervous arrangement past fashion of electrical and chemical signals. Chemic signaling occurs at the interface between different neurons when one of the cells releases molecules (called neurotransmitters) that diffuse across the modest gap between the cells (chosen the synapse) and bind to the surface of the other jail cell. These neurotransmitter molecules are stored in small intracellular structures called vesicles that fuse to the cell wall and then break open up to release their contents when the neuron is appropriately stimulated. This process is called exocytosis (see Figure 9). One neurotransmitter that has been very extensively studied is dopamine, C8H11NO2. Dopamine is involved in various neurological processes that touch a wide multifariousness of human being behaviors. Dysfunctions in the dopamine systems of the brain underlie serious neurological diseases such as Parkinson's and schizophrenia.
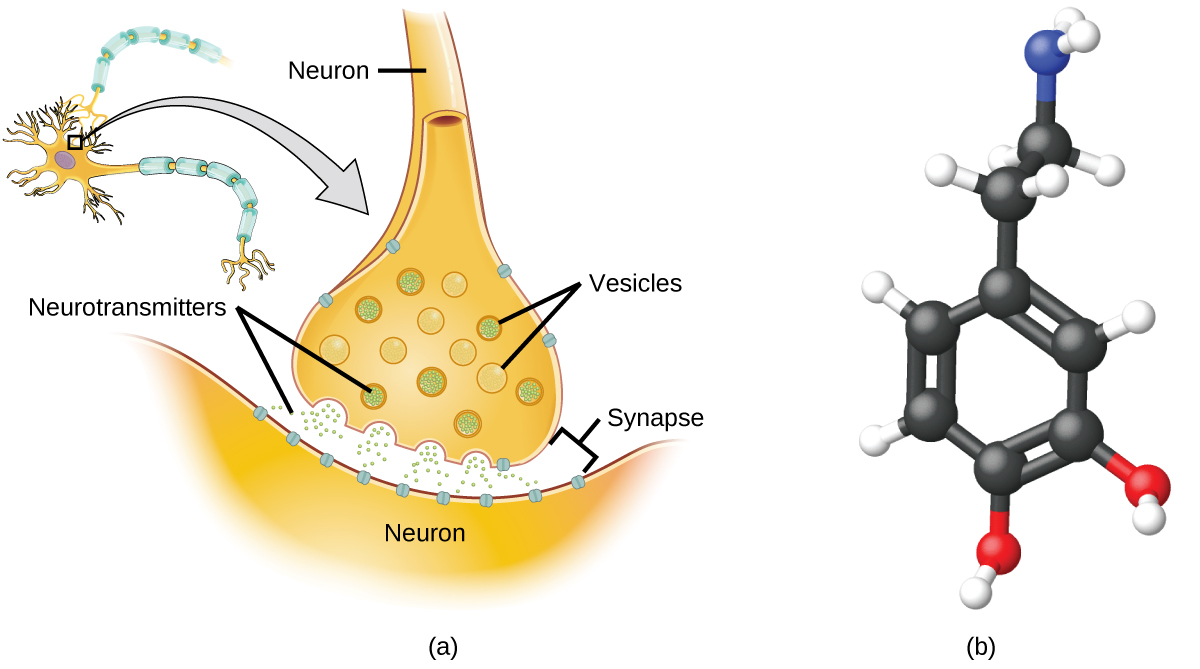
Ane of import aspect of the circuitous processes related to dopamine signaling is the number of neurotransmitter molecules released during exocytosis. Since this number is a primal cistron in determining neurological response (and subsequent human being thought and action), information technology is of import to know how this number changes with certain controlled stimulations, such as the administration of drugs. Information technology is also important to understand the mechanism responsible for whatsoever changes in the number of neurotransmitter molecules released—for instance, some dysfunction in exocytosis, a alter in the number of vesicles in the neuron, or a alter in the number of neurotransmitter molecules in each vesicle.
Significant progress has been fabricated recently in directly measuring the number of dopamine molecules stored in private vesicles and the corporeality really released when the vesicle undergoes exocytosis. Using miniaturized probes that can selectively discover dopamine molecules in very modest amounts, scientists have determined that the vesicles of a certain blazon of mouse brain neuron incorporate an boilerplate of 30,000 dopamine molecules per vesicle (near 5 × 10−20 mol or l zmol). Analysis of these neurons from mice subjected to various drug therapies shows significant changes in the boilerplate number of dopamine molecules contained in private vesicles, increasing or decreasing by upwardly to iii-fold, depending on the specific drug used. These studies too indicate that not all of the dopamine in a given vesicle is released during exocytosis, suggesting that it may be possible to regulate the fraction released using pharmaceutical therapies.[1]
Fundamental Concepts and Summary
The formula mass of a substance is the sum of the average diminutive masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent chemical compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental measurements have determined the number of entities composing 1 mole of substance to exist 6.022 × 1023, a quantity called Avogadro'south number. The mass in grams of i mole of substance is its tooth mass. Due to the use of the same reference substance in defining the atomic mass unit and the mole, the formula mass (amu) and molar mass (thousand/mol) for any substance are numerically equivalent (for instance, one HiiO molecule weighs approximately eighteen amu and 1 mole of HtwoO molecules weighs approximately 18 g).
Chemistry End of Affiliate Exercises
- What is the total mass (amu) of carbon in each of the following molecules?
(a) CH4
(b) CHCliii
(c) C12HxO6
(d) CHiiiCH2CHiiCH2CH3
- What is the full mass of hydrogen in each of the molecules?
(a) CH4
(b) CHCl3
(c) C12HtenO6
(d) CHthreeCH2CHtwoCH2CH3
- Calculate the molecular or formula mass of each of the following:
(a) P4
(b) H2O
(c) Ca(NOthree)ii
(d) CHthreeCO2H (acetic acid)
(e) C12H22O11 (sucrose, cane sugar).
- Determine the molecular mass of the post-obit compounds:
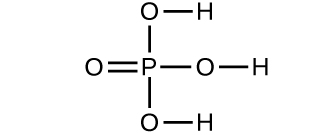
(a)
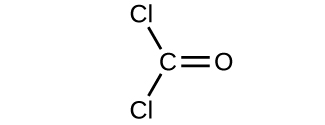
(b)
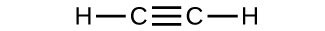
(c)
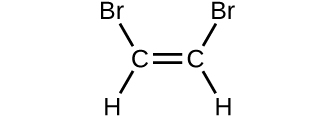
(d)
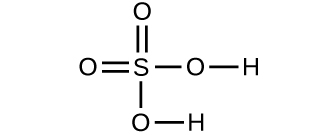
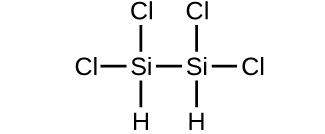
- Make up one's mind the molecular mass of the following compounds:
(a)
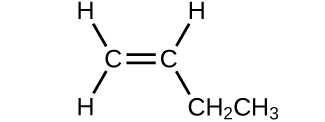
(b)
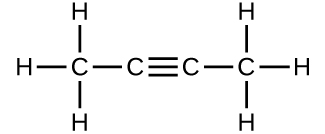
(c)
(d)
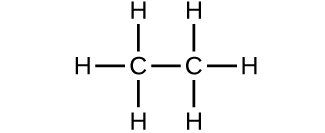
- Which molecule has a molecular mass of 28.05 amu?
(a)
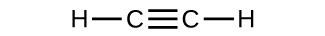
(b)
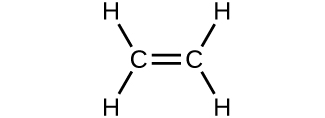
(c)
- Write a sentence that describes how to make up one's mind the number of moles of a compound in a known mass of the chemical compound if we know its molecular formula.
- Compare 1 mole of Htwo, 1 mole of O2, and 1 mole of F2.
(a) Which has the largest number of molecules? Explain why.
(b) Which has the greatest mass? Explain why.
- Which contains the greatest mass of oxygen: 0.75 mol of ethanol (C2HfiveOH), 0.sixty mol of formic acrid (HCOiiH), or 1.0 mol of water (HiiO)? Explicate why.
- Which contains the greatest number of moles of oxygen atoms: i mol of ethanol (CtwoHfiveOH), one mol of formic acid (HCOiiH), or 1 mol of water (H2O)? Explain why.
- How are the molecular mass and the molar mass of a compound like and how are they dissimilar?
- Calculate the tooth mass of each of the post-obit compounds:
(a) hydrogen fluoride, HF
(b) ammonia, NHthree
(c) nitric acid, HNOthree
(d) silver sulfate, Ag2SOiv
(e) boric acid, B(OH)3
- Calculate the molar mass of each of the following:
(a) Sviii
(b) C5H12
(c) Sc2(Then4)3
(d) CHthreeCOCH3 (acetone)
(e) C6H12O6 (glucose)
- Calculate the empirical or molecular formula mass and the molar mass of each of the following minerals:
(a) limestone, CaCOthree
(b) halite, NaCl
(c) beryl, Be3AltwoSisixO18
(d) malachite, Cu2(OH)2CO3
(e) turquoise, CuAlsix(PO4)4(OH)8(HiiO)4
- Calculate the molar mass of each of the following:
(a) the anesthetic halothane, C2HBrClFiii
(b) the herbicide paraquat, C12H14NtwoCl2
(c) caffeine, CviiiH10Due northivO2
(d) urea, CO(NHtwo)two
(due east) a typical soap, C17H35CO2Na
- Make up one's mind the number of moles of compound and the number of moles of each blazon of atom in each of the following:
(a) 25.0 k of propylene, C3Hsix
(b) 3.06 × 10−iii g of the amino acid glycine, C2H5NOtwo
(c) 25 lb of the herbicide Treflan, CthirteenH16Northward2O4F (ane lb = 454 g)
(d) 0.125 kg of the insecticide Paris Green, Cufour(AsOiii)2(CH3CO2)2
(e) 325 mg of aspirin, C6H4(CO2H)(COtwoCH3)
- Determine the mass of each of the following:
(a) 0.0146 mol KOH
(b) x.2 mol ethane, C2H6
(c) 1.6 × x−3 mol Na2 And so4
(d) 6.854 × 10three mol glucose, Csix H12 O6
(e) 2.86 mol Co(NHthree)6Cl3
- Decide the number of moles of the compound and determine the number of moles of each type of cantlet in each of the following:
(a) 2.12 m of potassium bromide, KBr
(b) 0.1488 g of phosphoric acid, H3PO4
(c) 23 kg of calcium carbonate, CaCOthree
(d) 78.452 k of aluminum sulfate, Al2(Soiv)three
(east) 0.1250 mg of caffeine, CeightHxN4O2
- Make up one's mind the mass of each of the following:
(a) 2.345 mol LiCl
(b) 0.0872 mol acetylene, C2H2
(c) 3.three × 10−two mol Na2 CO3
(d) 1.23 × ten3 mol fructose, Cvi H12 O6
(e) 0.5758 mol FeSOiv(HiiO)7
- The approximate minimum daily dietary requirement of the amino acid leucine, C6H13NO2, is one.one g. What is this requirement in moles?
- Make up one's mind the mass in grams of each of the following:
(a) 0.600 mol of oxygen atoms
(b) 0.600 mol of oxygen molecules, O2
(c) 0.600 mol of ozone molecules, O3
- A 55-kg woman has 7.5 × 10−iii mol of hemoglobin (tooth mass = 64,456 m/mol) in her claret. How many hemoglobin molecules is this? What is this quantity in grams?
- Determine the number of atoms and the mass of zirconium, silicon, and oxygen plant in 0.3384 mol of zircon, ZrSiOiv, a semiprecious stone.
- Make up one's mind which of the post-obit contains the greatest mass of hydrogen: one mol of CHiv, 0.six mol of CviH6, or 0.iv mol of C3Height.
- Determine which of the following contains the greatest mass of aluminum: 122 g of AlPOfour, 266 k of Al2C1half-dozen, or 225 grand of AltwoS3.
- Diamond is 1 form of elemental carbon. An engagement ring contains a diamond weighing ane.25 carats (ane carat = 200 mg). How many atoms are present in the diamond?
- The Cullinan diamond was the largest natural diamond ever plant (January 25, 1905). It weighed 3104 carats (1 carat = 200 mg). How many carbon atoms were present in the rock?
- One 55-gram serving of a particular cereal supplies 270 mg of sodium, xi% of the recommended daily allowance. How many moles and atoms of sodium are in the recommended daily allowance?
- A sure nut crunch cereal contains 11.0 grams of sugar (sucrose, C12H22O11) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of saccharide?
- A tube of toothpaste contains 0.76 g of sodium monofluorophosphate (NatwoPO3F) in 100 mL.
(a) What mass of fluorine atoms in mg was present?
(b) How many fluorine atoms were present?
- Which of the following represents the least number of molecules?
(a) 20.0 g of H2O (18.02 thou/mol)
(b) 77.0 yard of CH4 (16.06 g/mol)
(c) 68.0 g of CaHtwo (42.09 yard/mol)
(d) 100.0 g of N2O (44.02 g/mol)
(e) 84.0 thou of HF (twenty.01 m/mol)
Glossary
- Avogadro's number (NA )
- experimentally determined value of the number of entities comprising 1 mole of substance, equal to 6.022 × 1023 mol−1
- formula mass
- sum of the boilerplate masses for all atoms represented in a chemical formula; for covalent compounds, this is too the molecular mass
- molar mass
- mass in grams of 1 mole of a substance
- mole
- amount of substance containing the aforementioned number of atoms, molecules, ions, or other entities as the number of atoms in exactly 12 grams of 12C
Solutions
Answers to Chemistry Finish of Chapter Exercises
1. (a) 12.01 amu; (b) 12.01 amu; (c) 144.12 amu; (d) lx.05 amu
3. (a) 123.896 amu; (b) 18.015 amu; (c) 164.086 amu; (d) 60.052 amu; (due east) 342.297 amu
5. (a) 56.107 amu; (b) 54.091 amu; (c) 199.9976 amu; (d) 97.9950 amu
vii. Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the chemical compound expressed in grams.
9. Formic acrid. Its formula has twice as many oxygen atoms as the other two compounds (one each). Therefore, 0.threescore mol of formic acid would be equivalent to i.20 mol of a compound containing a single oxygen cantlet.
xi. The ii masses have the aforementioned numerical value, simply the units are different: The molecular mass is the mass of i molecule while the molar mass is the mass of 6.022 × ten23 molecules.
thirteen. (a) 256.528 chiliad/mol; (b) 72.150 1000 mol−1; (c) 378.103 g mol−i; (d) 58.080 1000 mol−1; (eastward) 180.158 g mol−1
15. (a) 197.382 g mol−1; (b) 257.163 grand mol−ane; (c) 194.193 g mol−i; (d) 60.056 thou mol−1; (due east) 306.464 one thousand mol−i
17. (a) 0.819 g; (b) 307 m; (c) 0.23 g; (d) 1.235 × 106 one thousand (1235 kg); (e) 765 thousand
19. (a) 99.41; (b) ii.27 thousand; (c) 3.5 g; (d) 222 kg; (due east) 160.1 g
21. (a) 9.lx g; (b) 19.ii g; (c) 28.8 g
23. zirconium: 2.038 × x23 atoms; 30.87 g; silicon: two.038 × 1023 atoms; 9.504 yard; oxygen: 8.151 × ten23 atoms; 21.66 thousand
25. AlPO4: ane.000 mol
AliiCl6: 1.994 mol
AltwoSouththree: iii.00 mol
27. 3.113 × x25 C atoms
29. 0.865 servings, or near i serving.
31. xx.0 g HiiO represents the to the lowest degree number of molecules since it has the least number of moles.
Source: https://opentextbc.ca/chemistry/chapter/3-1-formula-mass-and-the-mole-concept/
Posted by: pitrefith1963.blogspot.com

0 Response to "How Many Atoms Of Potassium Makeup 2 Mole"
Post a Comment